Global Pyrogen Testing Market is valued at USD 752.9 Million in 2018 and expected to reach USD 1749.4 Million by 2025 with a CAGR of 12.8% over the forecast period. Increasing prevalence of chronic diseases and growing awareness regarding healthcare are anticipated to drive growth of Global Pyrogen Testing Market.
Medical devices are tested for biocompatibility to protect patients from biological risks that may arise from their use and also in the quality assurance of medical products, tests for sterility are essential. It is crucial for parenteral pharmaceuticals to avoid the presence of pyrogens. Fever is one of the biological risks for which devices are screened. The term pyrogen defines fever-inducing substances. These kind of substances are not eliminated by standard sterilisation processes and become biologically active once enter into the bloodstream. They can cause risks to human health, ranging from mild reactions (e.g. fever) to septic shock and death. Therefore, for injectable formulations, pyrogen testing is mandatory. The increasing growth of medical device industry and pharmaceutical industry is anticipated to foster the demand for pyrogen testing. The pyrogenicity can be measured by using three kinds of tests which are commonly used; the in vivo rabbit pyrogen test (RPT), in vivo limulus amebocyte lysate (LAL) assay, and the in vitro monocyte activation test (MAT).
Scope of Global Pyrogen Testing Market Reports
Global Pyrogen Testing Market report is segmented on the basis of product, end-user, test and region & country level. Based upon product, global pyrogen testing market is classified into kits and reagents, instruments and services. Based upon end-user, global pyrogen testing market is divided into pharmaceuticals and biologics, medicals devices manufacturing and others. Based on test, the pyrogen testing market is classified into LAL test, in vitro pyrogen test and rabbit test.
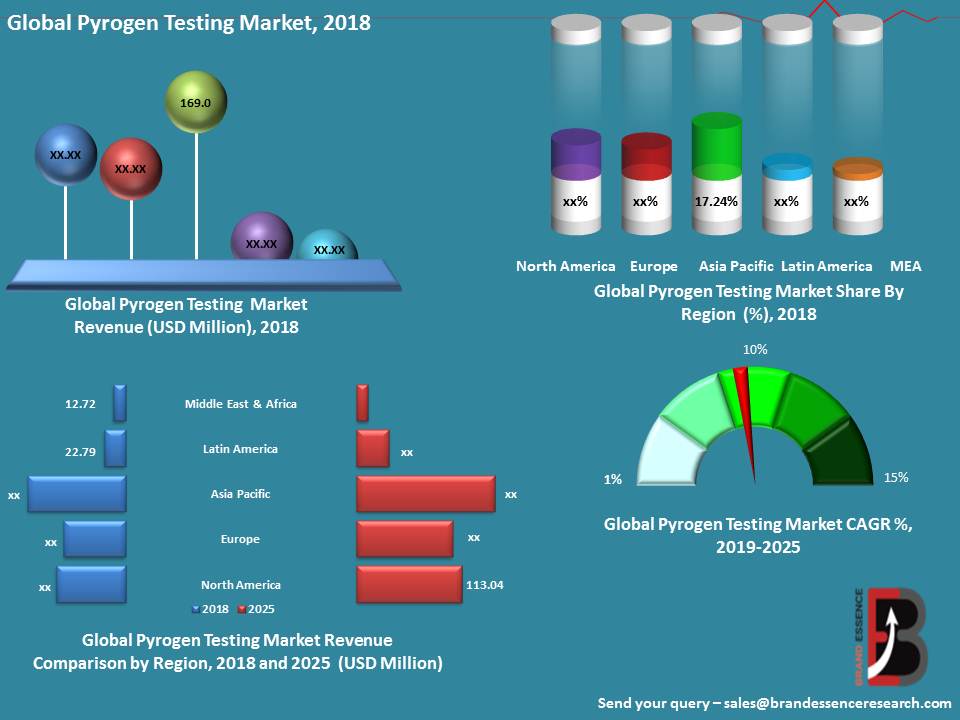
North America is Expected to Dominate the Global Pyrogen Testing Market
The global pyrogen testing market is segmented into North America, Europe, Asia-Pacific Latin America and Middle East & Africa. North America is expected to dominate the global pyrogen testing market within the forecast period attributed to the highly developed healthcare infrastructure and increasing investment by government in healthcare and pharmaceutical industry in this region. In addition, presence of leading players and high research and development activities of new drugs are also supplementing the growth of the pyrogen testing market in the region. Europe is projected to capture the second largest share of global pyrogen testing market owing to the increasing geriatric population in this region. Asia Pacific is anticipated to witness a significant growth in global pyrogen testing market owing to the improving healthcare infrastructure, growing awareness regarding healthcare and emerging opportunities in this region.
The regions covered in this Global Pyrogen Testing Market report are North America, Europe, Asia-Pacific and Rest of the World. On the basis of country level, the market of pyrogen testing is sub divided into U.S., Mexico, Canada, U.K., France, Germany, Italy, China, Japan, India, South East Asia, Middle East Asia (UAE, Saudi Arabia, Egypt) GCC, Africa, etc.
Key Players for Global Pyrogen Testing Market Report
Some major key players for Global Pyrogen Testing Market are Genscript, Sigma-Aldrich Corporation, Cape Cod Inc., Hyglos GMBH, Merck & Co. Inc., Ellab A/S, Wako Chemicals USA Inc., Lonza Group, Charles River Laboratories International Inc., and Thermo Fisher Scientific Inc. and others.
A pyrogen is a foreign substance that evokes temperature elevation in an animal’s body. Typically, pyrogenic substances include endotoxin and other bacterial by products. Pyrogen detection is mandatory in pharmaceutical, biotechnology or medical devices industries in order to avoid the fever reactions that can be induced by both microbial and non-microbial entities. So, during the study of Global Pyrogen Testing market, we have considered pyrogen testing products and consumables to analyze the market.Global pyrogen testing market report is segmented on the basis of product, test, application and regional & country level. Based on product global pyrogen testing market is classified as instruments, consumables and services. Based upon type, global pyrogen testing market is classified as LAL (Limulus Amebocyte Lysate) tests, In vitro tests and Rabbit tests. Based upon application type, global pyrogen testing market is classified as pharmaceutical & biologics, medical devices, diagnostics and others. The regions covered in this pyrogen testing market report are North America, Europe, Asia-Pacific and Rest of the World. On the basis of country level, market of Pyrogen Testing is sub divided into U.S., Mexico, Canada, U.K., France, Germany, Italy, China, Japan, India, South East Asia, GCC, Africa, etc.
Global Pyrogen Testing Market Dynamics
Rapidly growing pharmaceutical, biotechnology and medical devices industry, increasing Research & Development investments, favorable government policies, increasing number of new biologics products are driving the growth of the world pyrogen testing market. However, increasing application for animal-free tests and availability of other molecular diagnostics methods are major restrains of the Global Medical Robotic Market. Nonetheless, untapped market and technological advancements may generate new opportunities in forecast period.
Global Pyrogen Testing Market Regional Analysis
North America dominates the pyrogen testing market with highest market share due to presence of many large biotechnology and biopharmaceutical companies such as Pfizer Inc., Merck Group, Celgene Corp., F. Hoffmann-La Roche AG and Amgen Inc. Moreover, highly developed healthcare & research infrastructure and large focus on new drug development are the major factors responsible for continuous growth of this market.
Europe is the second largest market for Pyrogen Testing due to high investments in research and development activities, favorable government policies, establishment of new biotechnology, biopharmaceutical and medical devices companies are the major factors for the growth of the pyrogen testing market in this region. In 2016 research-based pharmaceutical industries invested an estimated approximately USD 40,900 million in Research & Development in Europe.Asia Pacific pyrogen testing market is witnessed with strong growth rate majorly due advancements in drug discovery, development, and production, increasing research & development activities and growing pharmaceutical and biotechnology industries. Clinical research organizations are focusing on Asian countries for clinical trials. In February 2017, a National Reimbursement Drug List (NRDL) update added around 340 new drugs in the list. Among the local biotech companies based in China, there are approximately 800 innovative molecules in the pipeline, among which 70–80 are in phase III.
 Key Benefits for Global Pyrogen Testing Market Reports
Global Pyrogen Testing Market report covers in depth historical and forecast analysis.
Global Pyrogen Testing Market research report provides detail information about Market Introduction, Market Summary, Global market Revenue (Revenue USD), Market Drivers, Market Restraints, Market opportunities, Competitive Analysis, Regional and Country Level.
Global Pyrogen Testing Market report helps to identify opportunities in market place.
Global Pyrogen Testing Market report covers extensive analysis of emerging trends and competitive landscape.
Key Benefits for Global Pyrogen Testing Market Reports
Global Pyrogen Testing Market report covers in depth historical and forecast analysis.
Global Pyrogen Testing Market research report provides detail information about Market Introduction, Market Summary, Global market Revenue (Revenue USD), Market Drivers, Market Restraints, Market opportunities, Competitive Analysis, Regional and Country Level.
Global Pyrogen Testing Market report helps to identify opportunities in market place.
Global Pyrogen Testing Market report covers extensive analysis of emerging trends and competitive landscape.
Global Pyrogen Testing Market Segmentation
By Product:
• Kits and Reagents
• Instruments
• Services
By End-user:
• Pharmaceuticals and Biologics
• Medicals Devices Manufacturing
• Others
By Test:
• LAL Test(Turbidimetric Test, Chromogenic Test, Gel Clot Test)
• In Vitro Pyrogen Test
• Rabbit Test
North America
U.S.
Mexico
Canada
Europe
UK
France
Germany
Italy
Asia Pacific
China
Japan
India
Southeast Asia
Latin America
Brazil
The Middle East and Africa
GCC
Africa
Rest of Middle East and Africa
Interested in this report?
Get your sample now!


Key Benefits for Global Pyrogen Testing Market Reports Global Pyrogen Testing Market report covers in depth historical and forecast analysis. Global Pyrogen Testing Market research report provides detail information about Market Introduction, Market Summary, Global market Revenue (Revenue USD), Market Drivers, Market Restraints, Market opportunities, Competitive Analysis, Regional and Country Level. Global Pyrogen Testing Market report helps to identify opportunities in market place. Global Pyrogen Testing Market report covers extensive analysis of emerging trends and competitive landscape.




